

Boxed coding system is an efficient and automatic drug packaging management system. The system is mainly used for coding and management in the process of drug packaging, which can collect the detailed data of the drug electronic regulatory code of each level of packaging and the coding association information of different levels of packaging in real time and accurately. Through the accurate management and tracking of each drug logistics and information flow, the boxed coding system provides great convenience for pharmaceutical enterprises and consumers.
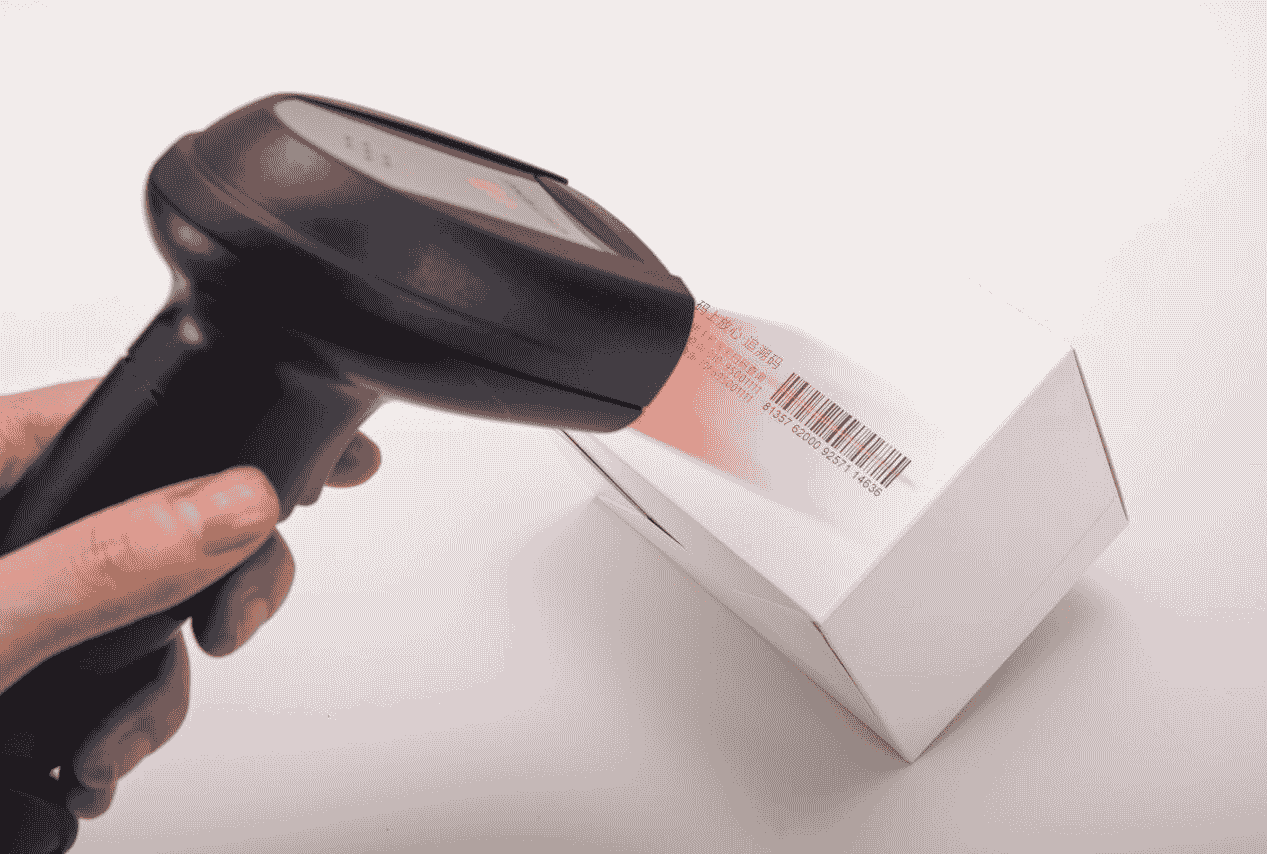
The workflow of automatic coding system mainly includes product processing, quality inspection audit, coding, packaging and delivery steps. The system assigns a unique identification number to each product and prints it on the product packaging, which helps to verify the authenticity of the product and obtain relevant information about the product, such as production date, shelf life, ingredients, etc. In addition, the system can also achieve product data collection, quality information management, production process traceability and other functions, through the production line product identification, positioning, coding and marking, to ensure product quality.
1. Improve drug safety: By adding two-dimensional code on drug packaging, consumers can easily and quickly scan two-dimensional code to obtain drug details, such as manufacturer, production batch, production date, etc., so as to ensure the safety of drugs.
2. Strengthen drug traceability: Drug two-dimensional code can realize the full traceability of drugs, from raw material procurement, production, storage, transportation and other links, to reduce the circulation of counterfeit and shoddy drugs.
3. Improve the transparency of the supply chain: The use of information technology means to achieve information sharing and transparency of the drug supply chain, and help relevant departments and enterprises to accurately supervise and manage drugs.
4. Legal compliance: The drug traceability code identification needs to meet the requirements of relevant national laws, regulations and standards, and the identification should be clear and readable, and can be read by code scanning equipment and human eyes.
5. Global standard implementation: To protect patient health and safety, serialization and traceability requirements are now standard for pharmaceutical products worldwide, such as the European Union's Anti-Counterfeit Pharmaceutical Directive and the U.S. Drug Supply Chain Security Act.
6. Full supervision and anti-counterfeiting: The purpose of online drug electronic supervision code is to give each drug product a unique ID card, through scanning collection uploaded to the super code system, users can query the entire circulation of the product through this unique bar code, so as to achieve the whole process of supervision, anti-counterfeiting, anti-transiting and so on.
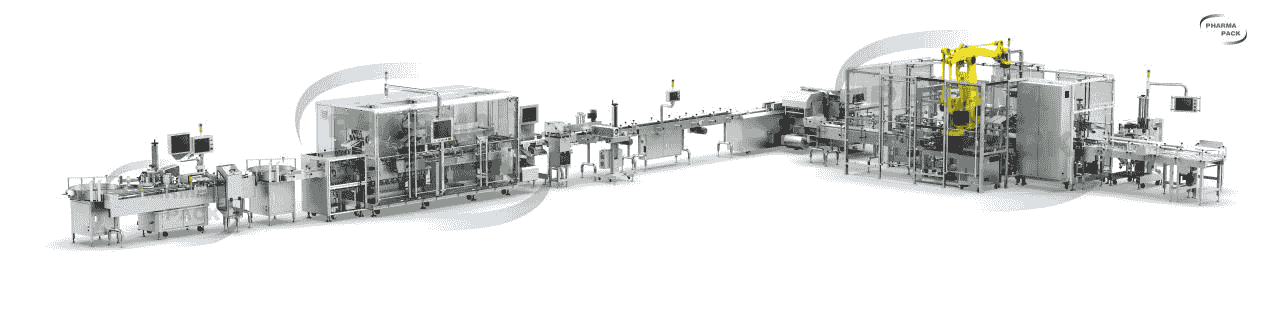
Pppharmapack box tracking system is composed of continuous box filling machine, box labeling resequencing machine and semi-automatic box packing machine, which can effectively complete the production process of box labeling, weighing detection, product serialization, packing into the box and box serialization. The system is compact in design, beautiful in shape and convenient in maintenance. Especially suitable for small space plant, can be customized according to the different needs of customers, widely used in medicine, health products, food, daily chemical and other industries.

In short, boxed coding system is of great significance in drug safety management and traceability. Through the introduction of advanced coding technology and management system, drug companies and consumers can more conveniently obtain drug information, improve drug safety, strengthen drug traceability, improve supply chain transparency, and achieve full supervision and anti-counterfeiting. With the implementation of global standards, boxed coding systems will play an increasingly important role in the pharmaceutical industry.