

Tablet inspection is a critical step in pharmaceutical manufacturing, ensuring product safety, integrity, and compliance. However, tablet inspection methods using manual operations or largely automated methods have certain limitations in effectively detecting tablet defects. Guaranteeing the integrity of tablets is critical for the safety and efficacy of pharmaceutical products. Pharmapack is an industry pioneer with the introduction of a groundbreaking solution, the Tablet Inspection System (TIS), redefining the standards for tablet inspection with innovation and efficiency. TIS can process on line defect detection for round/oval-shaped tablets and capsules.

Overview of Pharmapack's Tablet Inspection System (TIS)
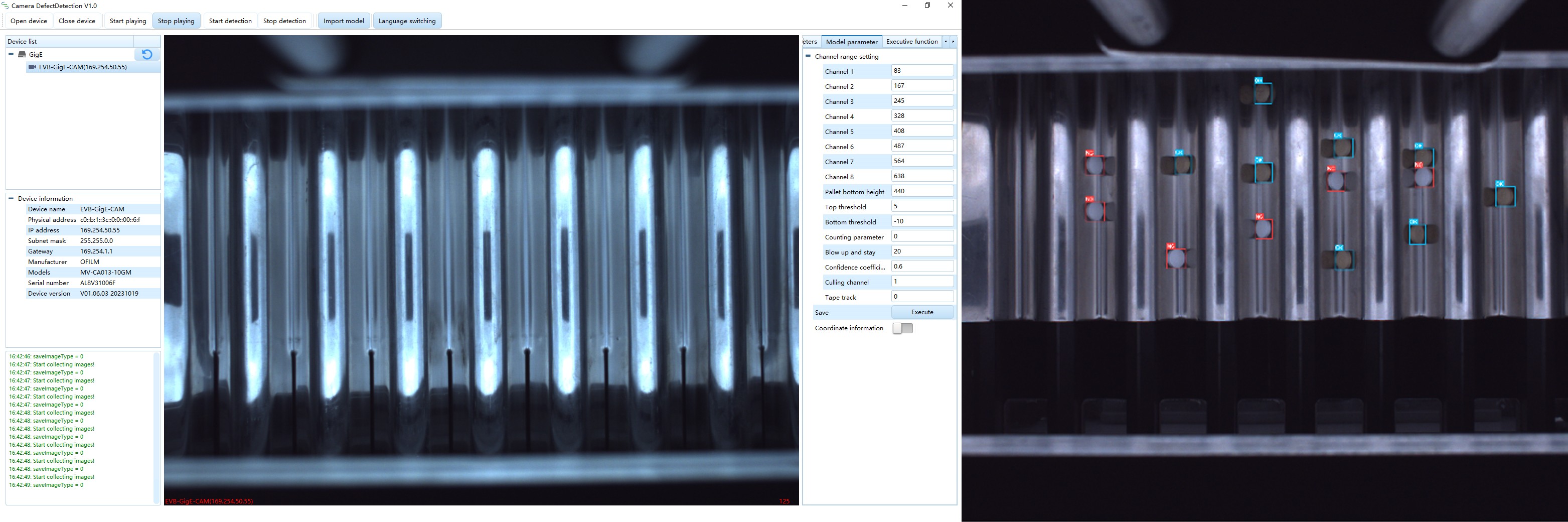
TIS is an advanced automated tablet inspection system that integrates high-resolution industrial cameras, precision industrial lenses, LED light sources, and advanced vision processors. This combination ensures optimal visibility and high-speed image processing, setting it apart from traditional tablet inspection machines.
During the production process, tablets pass through the TIS unit one by one via a conveyor belt or counter. High-resolution cameras mounted above and below the belt capture detailed images of each tablet, and precision LED lighting further enhances the visibility of the top and bottom surfaces of each tablet.
The images are processed at extremely high speeds by advanced vision processors and analyzed in depth using AI-driven software algorithms. These algorithms are trained to identify any defects and anomalies based on a library of images. Within a few milliseconds, any defective tablet will be flagged.
The TIS can be equipped with either a "Vacuum adsorption and removal" or a "Blow removal" option, which significantly reduces finished product loss compared to the traditional whole bottle reject mode.
1. Vacuum adsorption and removal
TIS utilizes a vacuum system to reject defective tablets. When a defect is discovered, the vacuum system comes into play, ensuring that defective tablets are removed without interrupting the production process.
2.Blow removal
This method employs high-speed and precise air-blowing technology to remove defective tablets. This rapid rejection mechanism ensures that only tablets that meet strict quality standards pass through the production line.
This automated inspection and rejection process enables 100% inspection of tablets and the elimination of substandard tablets without any human intervention.
Benefits of TIS in Pharmaceutical Manufacturing
Integrating TIS into the pharmaceutical manufacturing process provides numerous advantages. In addition to its core capabilities of high-speed processing and real-time data display, TIS also has the following benefits:
1) Improve product safety and integrity: TIS's advanced detection capabilities guarantee defect-free tablets, ensuring that each tablet meets the highest safety and integrity standards.
2) Comply with industry standards: Pharmaceutical regulations require strict quality control. TIS not only meets but exceeds these standards, offering a reliable solution for regulatory compliance in pharmaceutical manufacturing.
3) Enhanced real-time detection rate: TIS operates seamlessly in real-time, identifying and rejecting defective tablets at a speed that exceeds traditional inspection methods.
4) Reduce human error: By automating the inspection process, TIS significantly reduces the possibility of human error and helps to establish a more efficient quality control system.
The Need for Advanced Tablet Inspection
Traditional methods often face inherent challenges. Manual tablet inspection by operators is time-consuming, inconsistent, and prone to errors. While basic automation improves inspection efficiency, traditional systems still rely on 2D visible light cameras and are unable to detect the full range of surface and subsurface defects.
As regulatory requirements and quality standards in the pharmaceutical industry continue to rise, manufacturers require solutions that enable fast, accurate, and consistent in-line inspection of every tablet. Uncovering critical deficiencies is critical to ensuring that the final drug product meets safety and effectiveness guidelines. This is where Pharmapack's TIS comes in as the next generation of tablet quality control.
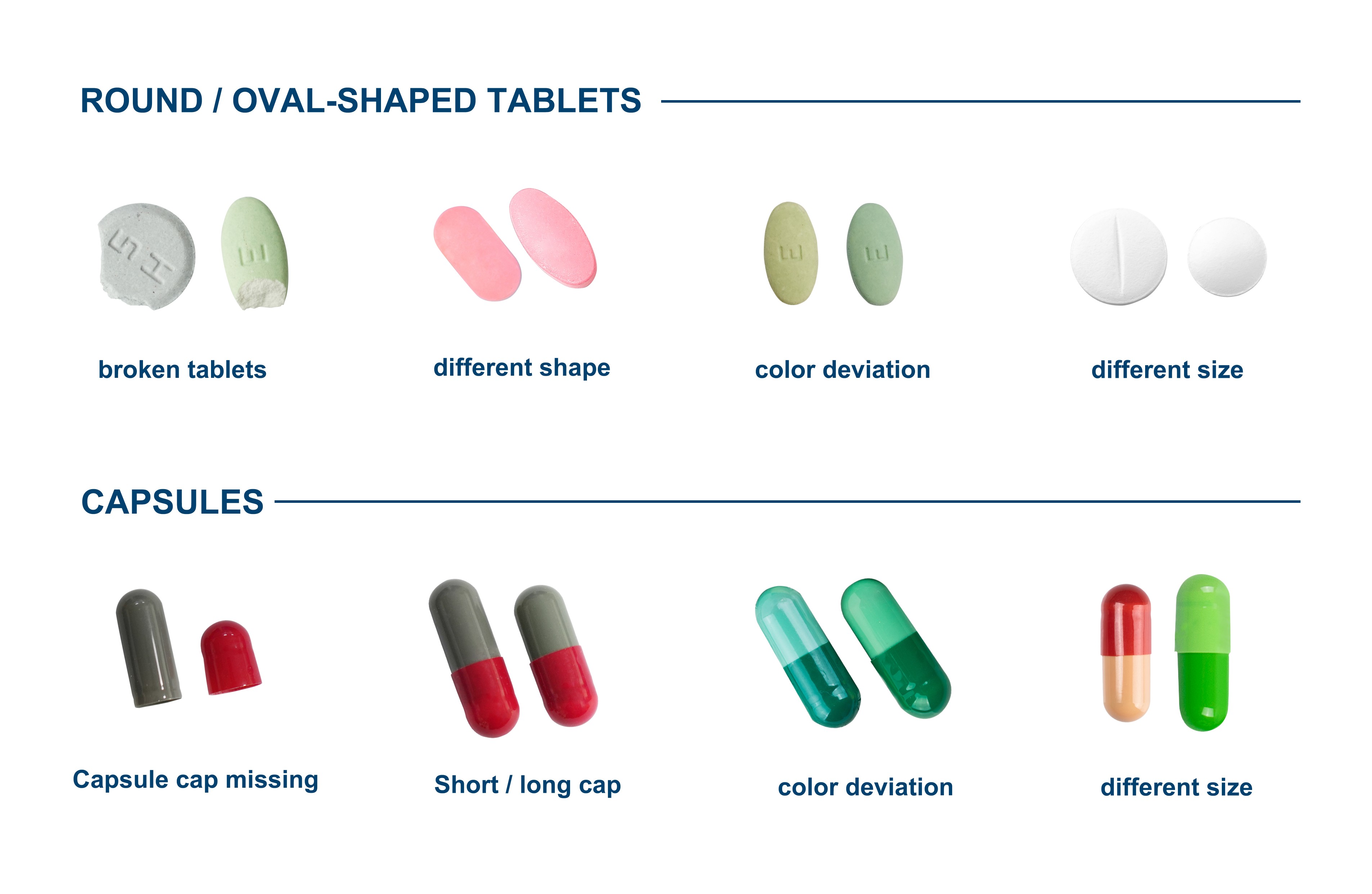
As regulatory scrutiny and consumer safety awareness increase, the pharmaceutical industry adopts advanced solutions such as TIS to ensure quality while earning the trust of consumers. Pharmapack ensures that every tablet meets the highest standards with accurate, reliable, and effective tablet inspection technology.
If you are interested in our product, please feel free to contact us!