

The pharmaceutical industry faces increasing pressures to ensure product safety, authenticity, and regulatory compliance. With counterfeit drugs becoming a growing concern and global supply chains becoming more complex, the importance of track and trace systems in pharmaceutical packaging has never been greater. These systems enable manufacturers to monitor the movement of pharmaceutical products from production to the point of sale, ensuring that patients receive safe, authentic medications.
At Pharmapack, we understand the challenges pharmaceutical manufacturers face in implementing track and trace solutions. Our advanced packaging solutions are designed to meet global regulatory requirements while improving efficiency, transparency, and security. In this article, we will explore the essential components of track and trace systems in pharmaceutical packaging, the challenges of implementation, and the latest technological advancements. We’ll also outline best practices for adoption, discuss the benefits of robust systems, and highlight future trends that will shape the industry.
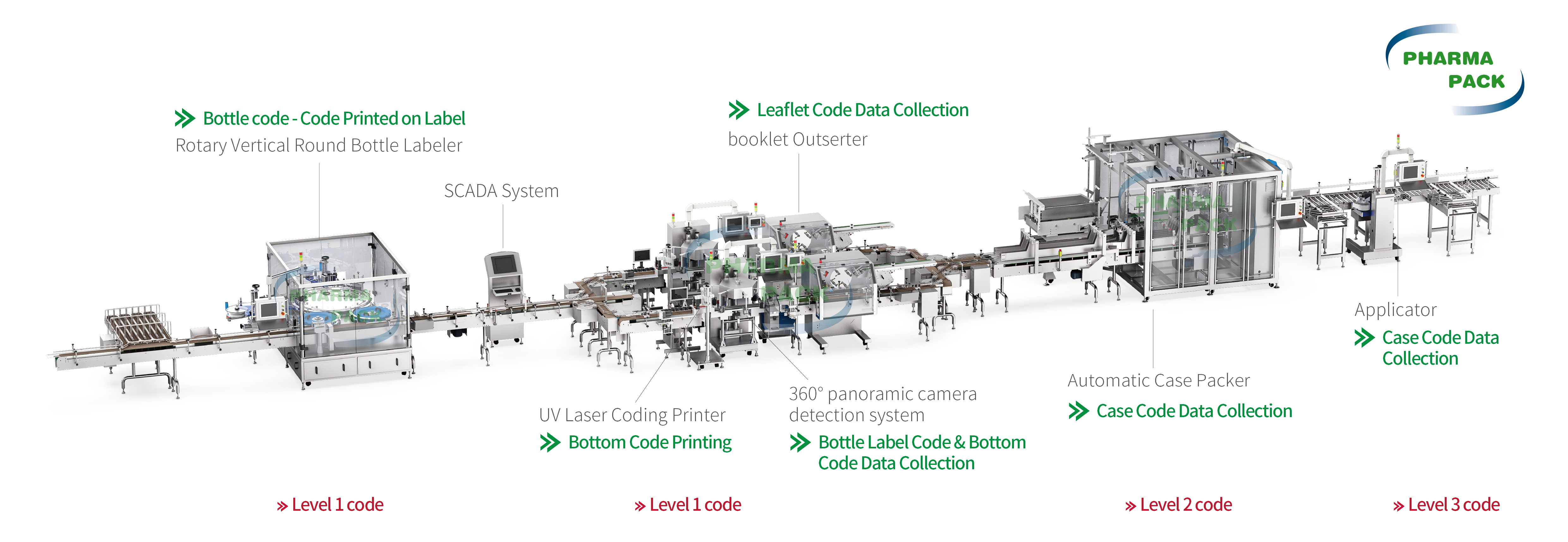
Track and trace in pharmaceutical packaging involves the use of technology to track a product's journey throughout the supply chain. The key components that make these systems effective are:
Serialization: This involves assigning a unique identifier, often a barcode, QR code, or RFID tag, to each product. Serialization ensures that every unit can be traced back to its source, enabling precise tracking throughout the supply chain.
Aggregation: While serialization tracks individual units, aggregation allows larger units, such as boxes or pallets, to be grouped while still maintaining traceability of the individual items inside. This is crucial for maintaining visibility across bulk shipments and ensuring product integrity.
Data Exchange: Effective track and trace systems rely on real-time data exchange between all stakeholders in the supply chain, including manufacturers, distributors, regulators, and even healthcare providers. This data sharing ensures complete transparency and helps mitigate the risk of counterfeit products entering the market.
The role of track and trace systems extends beyond regulatory compliance. By ensuring the safety and authenticity of pharmaceutical products, these systems help build trust with patients and healthcare providers, ultimately improving the reputation of pharmaceutical brands.
While the benefits of track and trace systems are clear, pharmaceutical companies often face several challenges when implementing them. These challenges, however, can be overcome with proper planning and the right technological solutions.
Regulatory Complexities: Pharmaceutical manufacturers must comply with a wide range of serialization and traceability regulations that vary from region to region. In the U.S., the Drug Supply Chain Security Act (DSCSA) mandates serialization, while the European Union’s Falsified Medicines Directive (FMD) requires additional measures. Navigating these regional differences can be complex, but Pharmapack’s solutions are designed to meet global standards, ensuring seamless compliance across multiple markets.
Operational Challenges: Integrating track and trace systems into existing packaging lines can be a significant operational challenge. Manufacturers often need to upgrade equipment, train staff, and adapt workflows to accommodate serialization and aggregation technologies. Pharmapack’s solutions are designed for easy integration, minimizing disruptions and ensuring smooth transitions.
Data Management: With track and trace systems generating vast amounts of data, managing and securely storing this information is critical. Effective data management ensures that product information is accurate, up-to-date, and compliant with regulations. Pharmapack’s cloud-based solutions provide secure, scalable data storage, allowing pharmaceutical companies to track products in real-time and ensure data integrity.
Technological innovations are continually enhancing the capabilities of track and trace systems. At Pharmapack, we stay at the forefront of these advancements to provide the most effective solutions for our clients.
Serialization Technology: Modern serialization technologies, such as 2D barcodes, QR codes, and RFID tags, enable manufacturers to track pharmaceutical products with unprecedented accuracy. These technologies not only allow for efficient tracking at the unit level but also enable real-time updates, enhancing supply chain transparency and efficiency.
Cloud-Based Solutions: Cloud computing has revolutionized track and trace systems by providing a centralized, secure platform for data storage and real-time information sharing. With cloud-based solutions, manufacturers can access up-to-date product data from anywhere in the world, ensuring that all stakeholders in the supply chain have the most current information. Pharmapack offers cloud solutions that meet the highest security standards, ensuring both compliance and ease of use.
Automation and AI: Automation and artificial intelligence (AI) are transforming the pharmaceutical packaging industry. Automation streamlines the processes of serialization and aggregation, reducing human error and increasing efficiency. Meanwhile, AI-driven tools help analyze the data generated by track and trace systems, offering insights that can predict potential supply chain disruptions and optimize operations. Pharmapack leverages these technologies to offer smarter, faster, and more reliable track and trace solutions.
To maximize the benefits of track and trace, pharmaceutical companies should follow best practices that ensure seamless implementation and ongoing efficiency.
Select the Right Technology: The first step is selecting the appropriate serialization and aggregation technology. It's essential to evaluate systems based on factors such as scalability, integration capabilities with existing ERP and MES systems, and ease of use. Pharmapack’s solutions are designed to integrate smoothly with existing workflows, minimizing disruption while ensuring scalability.
Ensure Regulatory Compliance: Adhering to local and global regulations is essential for avoiding fines and ensuring product safety. Pharmaceutical companies must ensure that their track and trace systems meet the requirements of key regulatory bodies such as the U.S. FDA and the European Medicines Agency. Pharmapack’s systems are built to comply with global standards, ensuring that our clients are always ahead of the regulatory curve.
Collaborate with Suppliers and Train Employees: Successful implementation requires collaboration with all stakeholders in the supply chain, including packaging machine manufacturers and contract manufacturing organizations (CMOs). Additionally, training employees is critical to ensure that everyone is familiar with the new system and processes. Pharmapack works closely with clients to ensure that staff are properly trained and that all systems are fully integrated.
A well-implemented track and trace system brings significant advantages to pharmaceutical companies, from operational improvements to enhanced brand trust.
Enhanced Recall Management: Track and trace systems make it easy to identify affected products quickly in the event of a recall. By providing real-time visibility into the movement of products, pharmaceutical companies can act swiftly to remove dangerous or compromised products from the market, protecting both patients and brand reputation.
Improved Supply Chain Transparency: With real-time tracking, companies gain unprecedented visibility into their supply chains. This transparency helps to identify inefficiencies, reduce delays, and prevent counterfeit products from entering the market. It also enables better decision-making, improving overall supply chain management.
Building Brand Trust: In an age of increased scrutiny over product safety, consumers and healthcare providers need assurance that the medications they are receiving are genuine. A well-implemented track and trace system helps demonstrate that commitment to quality and authenticity, ultimately strengthening brand reputation and increasing consumer confidence.
The future of track and trace in pharmaceutical packaging is brimming with exciting possibilities. Emerging technologies will continue to transform how products are tracked, monitored, and managed across the supply chain.
Blockchain Applications: Blockchain technology offers the potential for end-to-end traceability by providing an immutable, decentralized ledger of product movements. By integrating blockchain with track and trace systems, pharmaceutical companies can further enhance security and transparency, making it nearly impossible for counterfeit products to slip through the system.
IoT for Real-Time Monitoring: The Internet of Things (IoT) will enable real-time monitoring of pharmaceuticals during transit. Sensors embedded in packaging can track temperature, humidity, and other environmental factors, ensuring that products are stored and transported under optimal conditions. Pharmapack is already exploring IoT integration to offer even more comprehensive tracking capabilities.
AI for Predictive Analytics: Artificial intelligence will play an increasingly important role in the future of track and trace. By analyzing large volumes of data, AI can predict potential disruptions in the supply chain and recommend actions to prevent delays or issues. This predictive capability will help pharmaceutical companies stay ahead of potential problems, reducing operational downtime and ensuring consistent product delivery.
Track and trace systems are essential for ensuring the safety, compliance, and authenticity of pharmaceutical products. With the ever-increasing complexity of global supply chains and the need to combat counterfeit drugs, having a robust track and trace solution in place is more important than ever.
At Pharmapack, we offer cutting-edge track and trace solutions that not only meet the highest regulatory standards but also help pharmaceutical manufacturers optimize their operations. With innovations such as serialization, cloud-based platforms, and AI-powered tools, we are committed to helping our clients stay ahead of industry trends and ensure the integrity of their products at every stage of the supply chain.
As the pharmaceutical industry continues to evolve, investing in a comprehensive track and trace system will be crucial for maintaining compliance, improving efficiency, and building trust with consumers. Explore Pharmapack’s solutions today to ensure your business is prepared for the future of pharmaceutical packaging.